Knowing the Mole Ratio of a Reactant and Product
The mole ratio between H 2 and H 2 O is 11. We review their content and use your feedback to keep the quality high.

How To Find Moles Of Product From Moles Of Reactant Using A Chemical Equation Chemistry Study Com
Chemistry questions and answers.
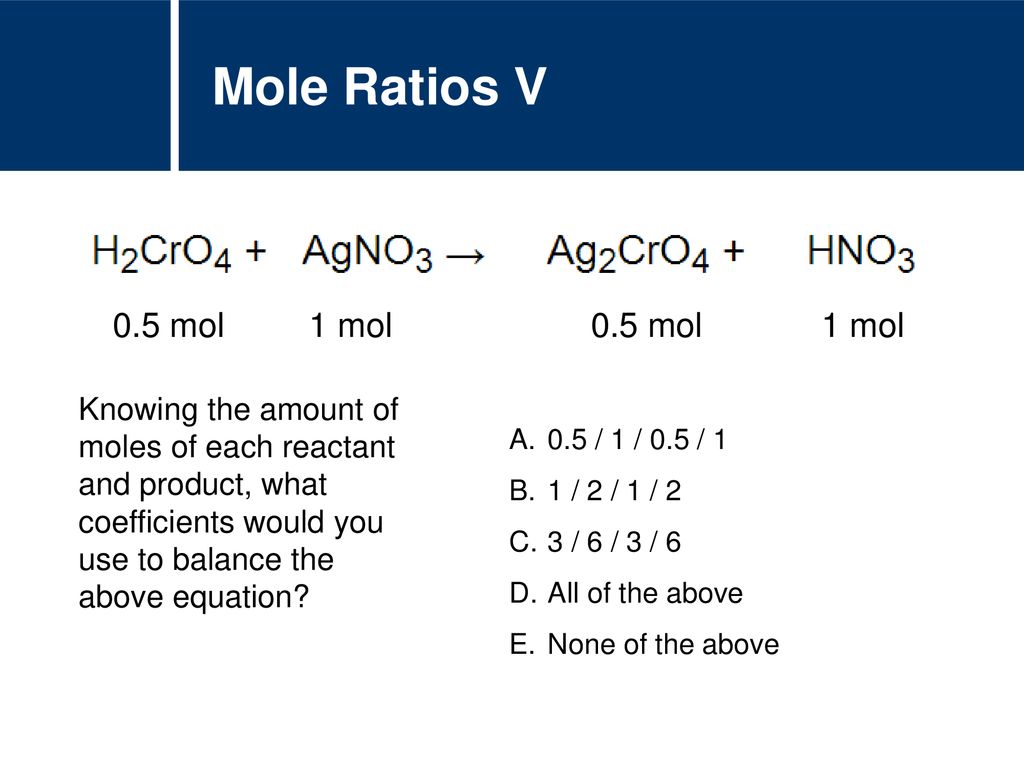
. Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine a. Whatever this value is the number of moles KNO3 is this valuemolar mass KNO3. To do mole ratio estimation in a reactant use this free online molar ratio calculator.
If 4 moles of hydrogen were used then 4 moles of water would be produced. LatexCH_4 2O_2 rightarrow CO_2 2H_2Olatex. The mole to mole ratio is determined by the coefficients of the chemical reactants and products example.
In a chemical reaction mole ratio is defined as the ratio of mole of one substance to the mole of another substance. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. For every 2 moles of H 2 used 2 moles of H 2 O are formed.
FeS 2HCl FeCl2 H2S Mol ratio 1 2 1 1 again. Experts are tested by Chegg as specialists in their subject area. Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations.
For example in this reaction. It is the limiting reactant that will react fully to directly determine the amount of product formed. 2AgNO3 aq K2CrO4 aq 2KNO3 aq Ag2CrO4 s Order custom essay Finding the Ratio of Mole of Reactants in a Chemical Reaction with free plagiarism report.
Mole Ratios Affect the Amount of the Product and the Amount of Each Reactant That Is Left Over. Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine. For example look at this equation.
Finding the Moles of Product from Moles of Reactant using a Chemical Equation. This model consists of five important principles as listed below see also Figure L171. Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine the mass of the product produced from a known mass of reactant For the reaction represented in the equation N23H2-2NH3 how many moles of nitrogen are required to produce 18 moles of ammonia.
The coefficients in a balanced chemical equation summarize the relative number of moles of each reactant and product involved in a chemical reaction. The speed of the reaction. In the equation 6CO2 6H2O C6H12O6 6O2 the mole ratio of water to oxygen is.
Convert any given data to number of moles Molar mass of. The energy released in the reaction. For every 1 mole of O 2 used 2 moles of H 2 O are formed.
The mass of the product produced from a known mass of reactant. Stoichiometric coefficients establish the mole ratio between reactants and products in a chemical reaction. This problem has been solved.
Take the ratio of the products stoichiometric coefficient and the. 3 mol H 2. Knowing the mole ratio of a reactant and product.
N 2 g 3 H 2 g Æ 2 NH 3 g The mole ratios between the molecules are. The speed of the reaction. Consider the following chemical equation.
The molar ratios identify how many moles of product are formed from a certain amount of reactant as well as the number of moles of a reactant needed to completely react with a certain amount of another reactant. If 010 moles of triglyceride react with an excess of NaOH how many moles of soap are predicted to form. 1 mol N 2.
If the reaction was CaCO 3 --- CO 2 CaO and you wanted to know the mass and number of moles of CaCO3 needed to make 44g of CO2 you would say that 1 mole CO2 comes from 1 mole of CaCO3. Using molar masses you can say that 12 16x2 or 44g of CO2 comes from 40. A very important key factor that leads you to know how to do limiting reactant calculations is the molar ratio of reactants with those of the products formed.
There is enough to make a valid conclusion because on the graph one can clearly see that the two lines intersect. Whether the reaction is reversible. Balance the Chemical Reaction.
703 assuming the reaction is determined by the reactants and the products on the other. 2 mol NH 3 The mole ratio means that 1 mol of N 2 will react with 3 mol of H 2 to form 2 mol of NH 3. Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine a.
Step 5 Sulphuric acid without conversion factors. The mass of the product produced from a known mass of reactant. The ratios of these coefficients represent the mole-to-mole or simply mole ratios that govern the disappearance of reactants and the appearance of products.
This is due to the mole ratio of sulphuric acid to aluminium sulphate being 3 moles to 1 mole which means the number of moles of sulphuric acid acting as a reactant is three times. Knowing the mole ratios in a. Multiply the amount in moles of aluminium sulphate by 3 in order to work out the number of moles of sulphuric acid needed as a reactant.
2Ag2O 4Ag O2 Mol ratio 24 1 notice. Who are the experts. In the equation 3CaOH2 3CaS04 the mole ratio of calcium.
NaClCaCl2 21 ratio therefore there will be twice as many moles since it takes one mole of reactant to create two moles of product as. Atomic theory is a model that has been developed over time to explain the properties and behavior of matter. Identify the theoretical molemole ratio of reactant to product for reaction B malet 1 maler o male react 2 males product 2 male 1 male product 2 malet radan 2 male product impossible to answer D Question 5 15 pts How will you know when enough acid has been added to the sodium bicarbonate in Reaction A or sodium.
General Latest Info. Zn s H2SO4 aq H2 g ZnSO4 aq energy a Name the products of this reaction. The mole ratio is used to compare the amount.
For example in the reaction. Thus one can also determine the mole ratio. You can find these particular ratios by using another mole fraction calculator as well.
Knowing the mole ratio of a reactant and product in a chemical reaction would allow you to determine what. Whether the reaction is reversible. In the equation Al2 SO43 3Ca OH2 3CaSO4 2Al OH3 the mole ratio of calcium hydroxide to aluminum hydroxide is.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. Hence the molar ratio is 1. 2 H 2 g O 2 g 2 H 2 O g The mole ratio between O 2 and H 2 O is 12.
The energy released in the reaction.

Converting Mass Of Reactant To Mass Of Product Examples Expii

How To Find The Mole Ratio And Molar Mass Youtube High School Chemistry Chemistry Lewis Acids And Bases

Determining The Mole Ratio Youtube

Simplified Method For Mole Conversions Chemistry Education Teaching Chemistry Mole Conversion

Aluminum Foil Limiting Reactants Stoichiometry Chemistry Lab Percent Yield Mod 8 Chemistry Labs Chemistry Lessons Stoichiometry Chemistry

How To Find The Mole Ratio And Molar Mass Youtube High School Chemistry Chemistry Lewis Acids And Bases

Cheat Sheet For Converting Between Moles And Grams And Atoms Teaching Chemistry Chemistry Education Chemistry Lessons

Chemistry Stoichiometry Mole Ratios Ppt Download

Question Video Determining The Molar Ratio Of Two Reactants Given The Mass And Molar Mass Nagwa

Stoichometry Problem Solving Organizer With Equations Problem Solving Solving Equations

Chem Awareness Reaction Stoichiometry Or Chemistry Stoichiometry Chemistry Chemical Equation Molar Mass

How To Find The Mole Ratio And Molar Mass Youtube High School Chemistry Chemistry Lewis Acids And Bases

Pin By Meganandjohn Deroos On Help Chemistry Lessons High School Chemistry Chemistry Classroom

Converting Moles Of Reactant To Moles Of Product Examples Expii

Mole Ratio Definition And Examples

Stoichiometry Cheat Sheet Mod 8 Teaching Chemistry Chemistry Lessons Study Chemistry
Stoichiometric Calculations Mole Ratio Easy Learning Youtube

Grade 11 Chemistry Stoichiometry Things To Know To Solve

Stoichiometry Mole To Mole Conversions Molar Ratio Practice Problems Youtube
Comments
Post a Comment